Gas Law includes Boyle’s law, Charles’s law, Gay-Lussac’s law and Avogador’s law
The gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between pressure, volume and temperature of a sample of gas could be obtained which would hold to approximation for all gases.
Boyle’s law
Charles's law, or the law of volumes, was found in 1787 by Jacques Charles. It states that, for a given mass of an ideal gas at constant pressure, the volume is directly proportional to its absolute temperature, assuming in a closed system. The statement of Charles's law is as follows: the volume (V) of a given mass of a gas, at constant pressure (P), is directly proportional to its temperature (T). As a mathematical equation, Charles's law is written as either:
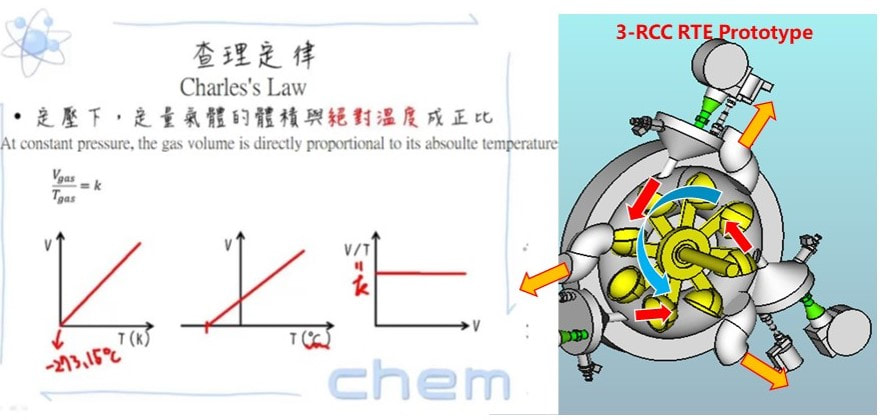
Rocket Turbine Engine (RTE) theory:
X 1
---------- = -----------
373.15 273.15
The X = 1.366, so from 0oC to 100oC, the gas volume increases to 1.366 = 36.6%
In gasoline combustion chamber, the temperature is about 1500 o C, so the gas volume increase to about 19 times. The RTE mechanical design takes the air gas explosion volume which is very powerful in the closed torus enclosure and to push the turbine blade forward until to the exhaust pipe.

RTE vs gas turbine engine:
A RTE efficiently applies most of the energy from gas explosion to rotate the turbine/shaft along the tangent line, which is the most efficient path. In contrast, a regular gas turbine engine, while capable of producing significant power and thrust, has the thermal efficiency only around 30% (used solely for shaft power) because the energy applies to the “face” of the turbine blades.
A RTE efficiently applies most of the energy from gas explosion to rotate the turbine/shaft along the tangent line, which is the most efficient path. In contrast, a regular gas turbine engine, while capable of producing significant power and thrust, has the thermal efficiency only around 30% (used solely for shaft power) because the energy applies to the “face” of the turbine blades.